
Service Agreements
Find out more about our Service Agreements here.

Curing diseases instead of merely treating symptoms – that’s the transformative promise of cell and gene therapies (CGTs). From autologous therapies like CAR-T cells to allogeneic cell therapies, the market is growing at a rapid pace. While most process steps in manufacturing are still performed manually today, biopharmaceutical manufacturers need to prepare now for commercial production. Syntegon is here to support you with matching equipment and seamless support throughout your product and project lifecycle.

With hundreds of cell and gene therapies in clinical trials, the CGT market is one of the most dynamic in the current pharmaceutical landscape. According to GlobalData, analysts forecast it will grow with an average of 40% p.a. between 2024 and 2030.
Of the more than 31,000 injectable products currently on the market (as of March 2025), biologics represent 12%. However, the picture shifts significantly when looking at the clinical pipeline: biologics now make up around 84% of the approximately 6,500 injectable therapies in development. Of these biologics, 34% fall under the category of cell and gene therapies. This group can be further divided into three segments: cell therapies (35%), gene therapies (16%), and gene-modified therapies (49%).

One of the main obstacles to the market adoption of cell and gene therapies is their high cost. Treatments such as CAR-T cell therapies often amount to several hundred thousand US dollars per patient, while altogether prices for CGTs can be as high as 4 million USD per treatment. These figures primarily reflect the substantial costs associated with development and manufacturing.
A major cost driver is the reliance on manual production processes. To reduce expenses and improve accessibility, scaling up and automating manufacturing is essential. This is key to making CGTs more cost-effective and facilitating broader patient access. A challenge that we at Syntegon are happy to tackle together with you.
Discover how Syntegon can support you in your cell and gene projects with seamless support along your product and project lifecycle – from consulting to fully automated equipment solutions and sustainable services.
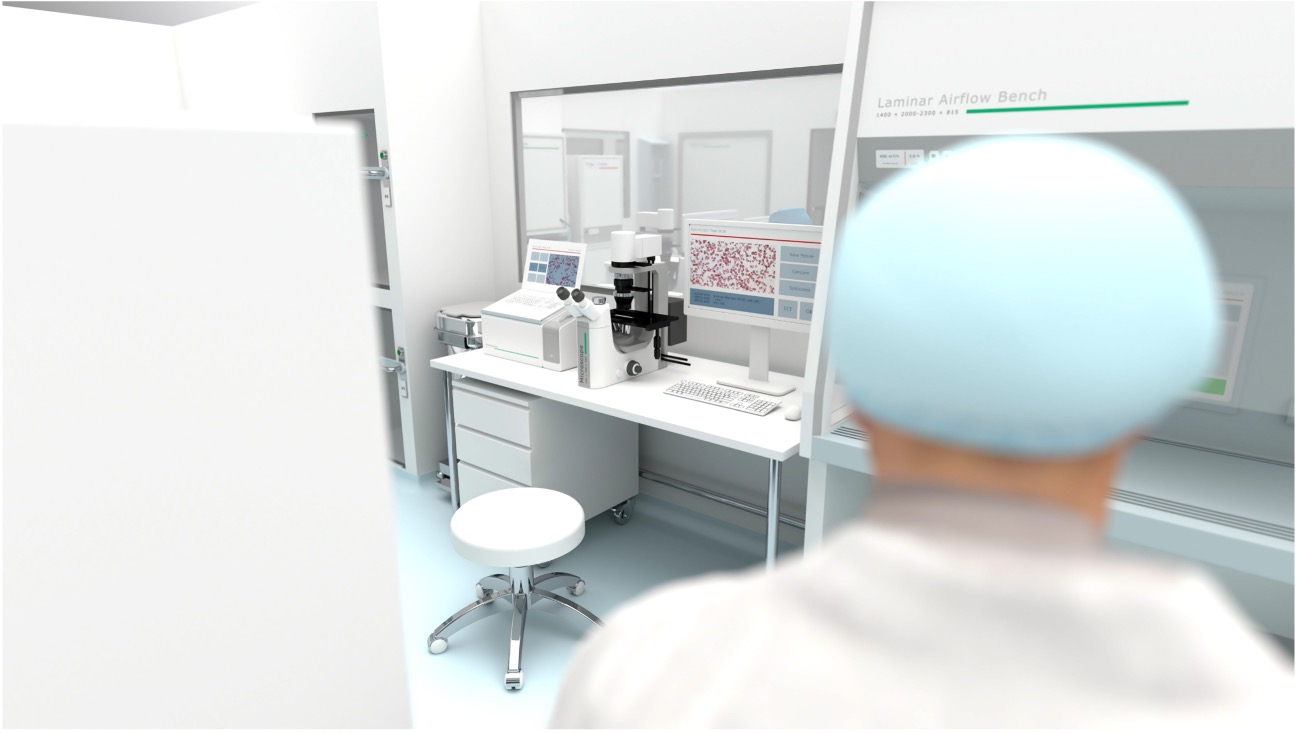




Syntegon is your ideal strategic partner for your cell and gene therapy manufacturing process. You receive seamless support along your entire product and project lifecycle – from consulting to equipment and services. Rely on our long-standing experience, global presence, and innovative technologies. Together, we will enhance the time to market of your cell and gene therapies.

Find out more about our Service Agreements here.

Find out more about Digital Solutions here.

Find out more about Parts here.

Find out more about Maintenance here.
/remote-assistant.jpg?width=640&height=380&name=remote-assistant.jpg)
Find out more about Technical Support here.

Find out more about Modernizations here.

Find out more about our Training here.

Find out more about our Expert Services here.

Syntegon is here to support you with matching equipment and seamless support throughout your product and project lifecycle.
Syntegon launches AIM9, combining visual inspection and leak detection with up to 600 vials per minute for pharma quality.
Discover how Syntegon enhances vial processes with expert formulation, filling, freeze-drying, and...
Discover solutions for regulatory challenges in vial filling operations. Enhance efficiency and...
A new PW generator enhances Vetter’s Langenargen site, ensuring reliable purified water supply and...
Discover how Vetter and Syntegon's led to the innovative Versynta microBatch, a fully automated,...