How does a North American CDMO grow from a small legacy facility into a world-class medium-sized facility? We highlight an example in the case study attached.
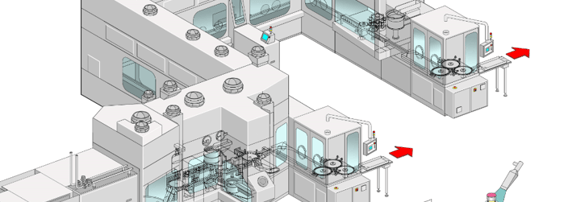
The overview includes the main drivers for this particular project, key considerations, line design, qualification measures, aseptic processing, and long-term experience.
Additionally, we will browse through the current state-of-the-art aseptic filling technology.
Benefits are insight into design concepts, selection process, sophisticated isolator equipment, and advanced aseptic technology.
There are multiple concepts that have become standard over the years.
Considerations for new projects:
- Catalytic converter combined with clever air recirculation for best bio-decontamination cycle-time results
- Line configurations for bulk containers or pre-sterilized nested vials, syringes, cartridges
- Point of use return air filter (direct in return air duct) for highly toxic applications
- Semi and fully automated wash down procedures
- Sterilizable aseptic valves in formulation / compounding application
- Fully integrated wireless glove testing system
- Highest flexibility of combi filling stations by using multiple – to specific liquid products – matching solutions
This article and the associated presentation were written by Juergen Metzger, Isolator and Process Specialist at Syntegon, formerly Bosch Packaging Technology.



