A major source of pollution in aseptic manufacturing is personal handling. Therefore, the reduction of human interventions in the critical zone leads to higher purity in the products.

At some point, every pharmaceutical manufacturer will face the never-ending discussion of RABS versus isolator technology. To ensure that the barrier system meets the needs of the particular product and facility, it is important to consider the different options available in this field. The two most common isolation technologies used by pharmaceutical companies are restricted access barrier systems (RABS) and isolators.
RABS: solution with focus on flexible handling
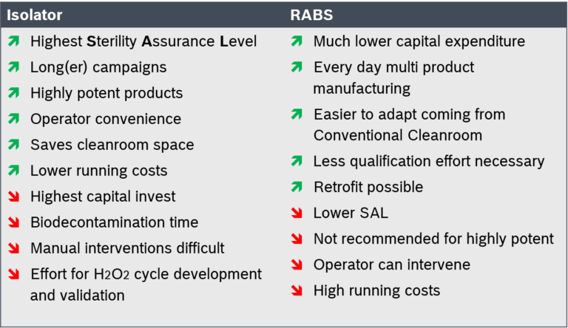
RABS provides a physical barrier between the production area and the operator environment. The production area has a rigid machine enclosure, safety-locked doors and ports with gloves. Depending on the kind of aeration, RABS can be divided into active and passive systems. Active RABS are equipped with dedicated air handling equipment, while passive RABS are sealed to the existing ceiling of a class B cleanroom. It is an attractive option for existing clean rooms to improve filling quality and for applications with higher flexibility requirements. If operated properly as an integrated system, RABS technology can approach the microbiological quality of an isolator. RABS generally have to be cleaned and decontaminated manually. Over the last couple of years, combinations with automated room biodecontamination can be seen more frequent. The biggest challenge for the operation of RABS, however, is that they require a highly classified ISO 5 environment.

Isolators: solution with highest sterility assurance levels
An isolator in turn is a completely sealed system with a complete separation of operator and process area. Doors cannot be opened during production, which makes it possible to operate isolators in a class C cleanroom environment (ISO 8, in operation). Pharmaceutical Isolators are typically equipped with a fully reproducible and validatable system for biodecontamination (mostly H2O2) and an process air handling unit (AHU) that ensures temperature control by heating or cooling, as well as permanent overpressure control of the process area compared to the operator environment in order to avoid ingress of contaminated air. Especially in terms of biodecontamination cycle times, huge improvements have been made to make a more flexible usage also for isolators possible.

Syntegon Newsletter
Get inspired with regular updates on technologies and services, case studies, webinar offers and events.
Comparison of Isolators and RABS
Both RABS as well as Isolator systems are well established in the pharmaceutical industry with hundreds of worldwide installations. If you think of differentiation factors, both quantitative as well as qualitative factors can be found. In terms of investment and operational costs, RABS offer the advantage of a low investment. On the other hand, running costs (e.g. gowning, environmental monitoring, etc.) are much higher compared to isolator systems. The initial investment of an isolator system is due to the dedicated biodecontamination as well as process air handling equipment on a higher level.
For each system, many customer specific elements highly affect the results of a comparative calculation. To find out which barrier system is most suited for a specific application and which technologies are needed to fulfill new regulatory requirements, it requires a reliable partner with extensive industry knowledge.
Syntegon, formerly Bosch Packaging Technology, has decades of experience in the design and manufacture of RABS, isolators and complete production lines, with references available from all over the world. We offer you a unique integration approach – ranging from the isolator itself, to the bio-decontamination and air-handling technology to the qualification and validation of the entire system - all from one source.